When Professor Bourdon learned of our organization, he contacted us to make sure we were aware of the results of an adverse-event survey of recipients of a 3rd dose of Pfizer's BNT162b2 conducted by Israel's Health Ministry. In his view, anyone examining survey data would conclude that the risks of boosters for college students outweigh the benefits, the result being “no college mandates” (at least for booster doses).
Professor Bourdon offered to write an essay “Questioning the Logic of COVID-Vaccine Mandates” for our Substack. Because mandates are illogical from many different perspectives, his essay wound up being quite long. Thus, we will publish it in two parts.
In this installment, Professor Bourdon raises and comments on the following three questions:
1. To what extent, if any, have those mandating COVID vaccines considered potential long-term risks?
2. Do risks of short-term side-effects diminish with each dose of a COVID vaccine?
3. Were justifications for COVID-vaccine mandates imposed on college and university students for the academic year 2021-2022 based on science or wishful thinking?
Questioning the Logic of COVID-Vaccine Mandates: Part I
As a mathematician, I have found many aspects of the USA's response to the coronavirus pandemic to be troublingly illogical. For instance, spring of 2020, wouldn't it have been logical for government agencies to begin funding and supporting investigations of potentially effective treatments of COVID-19 just as generously as they funded and supported vaccine development? Back in March 2020, there was absolutely no guarantee a vaccine could be developed “at warp speed'” or even at all—past attempts to develop coronavirus vaccines resulted in failure. Even if our public-health and political leaders knew in March 2020 a vaccine would be ready by December 2020, shouldn't they have wanted to treat effectively those infected March-December? Today, we know that many of the vaccinated also need effective treatments. Thus, in my view, the decision, made spring of 2020, not to devote significant resources to the testing of and development of effective treatments is not only illogical but also reckless, resulting in many preventable deaths from COVID-19.
I've also wondered why our political leaders who speak incessantly about following “the science” do not themselves follow decision science, which suggests that when policy makers wish to take advantage of expert opinion, they should never rely on the counsel of one expert; rather, a diverse group of experts should be consulted, the independence of their judgments should be preserved, and, roughly speaking, their perspectives should be averaged—see, e.g., the article“Intuitions About Combining Opinions: Misappreciation of the Averaging Principle,” whose final sentence reads, “The failure to combine judgments [of experts] comes at a high price in many common social and organizational settings.”
Here's another question I’ve contemplated: When did scientific truth become so obvious that the meaning of “following the science” is always clear? Those who would answer this question “Spring 2020” should consider the following:
[F]or many current scientific fields, claimed research findings may often be simply accurate measures of the prevailing bias.
From “Why Most Published Research Findings Are False” (published 30 August 2005 in PLOS Medicine) by John P. A. Ioannidis, currently Professor of Medicine at Stanford University
Most scientific studies are wrong, and they are wrong because scientists are interested in funding and careers rather than truth.
From a British Medical Journal opinion article (which appeared 9 September 2013) by Dr. Richard Smith, Editor of the BMJ, 1991-2004
Not breaking news: many scientific studies are ultimately proved wrong!.
Title of an article, published by The Guardian 17 Sep 2013, written in response to Richard Smith’s BMJ opinion article a link to which is provided above
I have many additional questions concerning pandemic-response logic, but I will focus here on six questions pertinent to the logic of mandating COVID vaccination—especially for college students.
1. To what extent, if any, have those mandating COVID vaccines considered potential long-term risks?
Anyone responsible for COVID vaccine mandates, as well as anyone supporting the mandates, should be concerned about the contents of Section 13.1 the FDA's “package insert” information relating to the risks associated with Pfizer’s COVID vaccine (Comirnaty):
Section 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
COMIRNATY has not been evaluated for the potential to cause carcinogenicity, genotoxicity, or impairment of male fertility. In a developmental toxicity study in rats with COMIRNATY there were no vaccine-related effects on female fertility [see Use in Specific Populations (8.1)].
Section 13.1 suggests that neither Pfizer nor the FDA has undertaken any analysis of potential long-term adverse health impacts of Pfizer’s COVID vaccine other than a toxicity study in female rats relating to female-rat fertility, fetal development, and postnatal development. No one can rule out the possibility that COVID-vaccination has long-term health effects predisposing those who receive it to develop certain illnesses later in their lives (e.g., cancer, MS, CJD), potentially ending their lives prematurely or impacting the quality of their lives for decades.
2. Do risks of short-term side-effects diminish with each dose of a COVID vaccine?
I believe most readers would agree that the answer to the preceding question is no—there is strong evidence suggesting that the second dose of the “primary series” of mRNA COVID-vaccination carries greater side-effect risks than does the first dose; e.g., data presented in the New England Journal of Medicine article reporting results of the phase III clinical trial for Pfizer's COVID vaccine BNT162b2 provides such evidence: Part B of Figure 2 shows that for 8 of the 9 categories of adverse events considered, the percentage of those experiencing the event post dose 2 exceeds the corresponding percentage post dose 1; moreover, the percentages reporting the adverse event to be severe tend to be higher following dose 2.
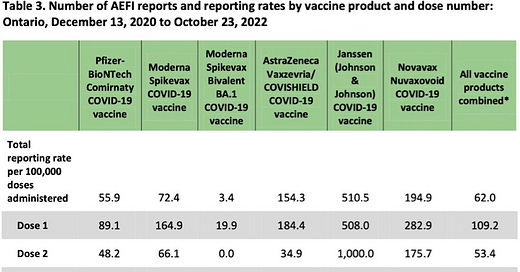
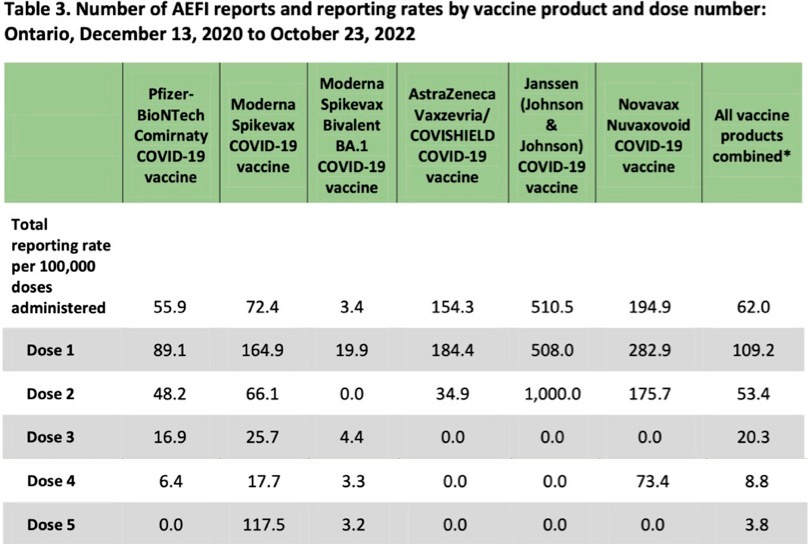
Those who might argue that yes is the answer to our question about diminishing side-effect risk with each dose would probably depend on data from a passive adverse-event reporting system like that used in Ontario. Here's some adverse-event data from a surveillance report provided by Public Health Ontario titled “Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario: December 13, 2020 to October 23, 2022”:
The preceding seems to offer strong evidence that our question does, in fact, have the answer yes—side-effect risks do diminish with each dose. However, owing to under-reporting in passive adverse-event data-collection systems (perhaps more likely after later doses owing to “reporting fatigue” or the realization that public-health agencies seem to be ignoring reports of AEFIs), the data in the table above certainly does not establish the answer to our question is yes.
We already noted that clinical-trial data suggests short-term side-effect risks for dose 2 exceed those of dose 1. Let's now consider some booster-dose adverse-event data that was actively collected and that suggests booter-dose adverse-event risks may exceed those of earlier doses.
Anyone considering mandating COVID vaccine booster doses or anyone supporting the mandating of booster doses should be thoroughly familiar with the results, discussed below, of an adverse-event survey of recipients of a 3rd dose of Pfizer's BNT162b2 conducted by Israel's Health Ministry. The Ministry describes the importance of the survey as follows:
From data collection by medical teams or self-reporting by the public of side-effects in temporal proximity (passive monitoring), it appears that there is underreporting; therefore, it is important to identify side-effects in temporal proximity to vaccination with the booster in an active manner via a dedicated survey.
A press release titled “Reported Adverse Events After Receiving Pfizer's Third (Booster) Dose of the Vaccine for COVID-19” was posted at the website of Israel's Ministry of Health on 9 February 2022. The press release reports on a survey designed “to review the frequency of side effects that appeared within 21 to 30 days after vaccination with Pfizer's third (booster) vaccine against COVID-19 among Israeli citizens aged 18 and older.” The survey sample was random and stratified by gender (male, female) and age group (18--39, 40--59, 60+). Responses from a total of 2,049 Israeli citizens to telephone interviews by experienced interviewers or research assistants constitute the survey's principal data. The following reassuring summary of survey information concludes the Israeli Health Ministry press release:
Most reported side effect [sic] of all kinds indicate that the onset of side effects after the third vaccination was not any worse than the first two vaccinations.
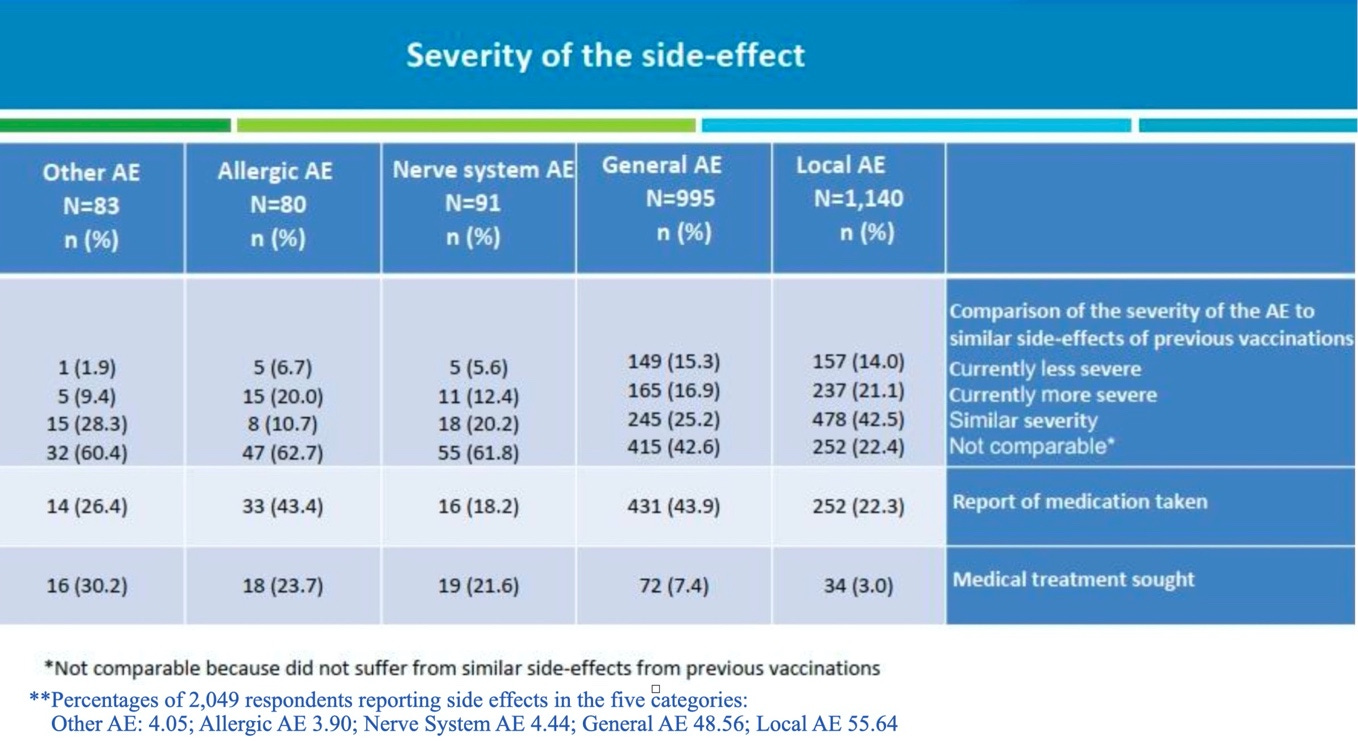
There is no link to survey data provided as part of the press release. As a general principle, we have reason to be skeptical of convenient summaries of data provided by press releases that do not provide access to source data. In this case, skepticism is justified. A Health Ministry survey-data report is available in Hebrew through Mivzaklive News. I found an English translation, but it's been removed. I have shared some pages from the translation below. If you don't know Hebrew and don't trust the translation, you can download the survey data via the Mivzaklive link above, copy the relevant text, and use Google Translate to translate to English. (I did that to check the translation.) Here's a copy of page 24 of the survey-data report comparing severity of adverse events (AEs) following the third dose versus earlier doses (with the footnote in blue added by me to provide context):
Table 1: Data from Israeli Health Ministry's Adverse Event Survey of Recipients of Dose 3 of Pfizer's BNT162b2
Recall that Israel's Health Ministry summarized the information contained in the preceding table as follows: “Most reported side effect [sic] of all kinds indicate that the onset of side effects after the third vaccination was not any worse than the first two vaccinations.” Review the data in the table carefully. How would you summarize it? I would summarize the responses as follows:
for third-dose side effects in every one of the five categories, of those respondents who experienced a similar side effect after a previous dose, a majority indicated the side effect to be similar in severity as that from a previous dose or more severe;
for four of the five categories of side effects (all but Local AEs), a majority reported either that they were currently experiencing a more severe case of a side effect previously encountered or were dealing with a new side effect, not experienced after a earlier dose.
The bottom line is that the Israeli Health Ministry's characterization of the data in the table above is clearly misleading. Consider the percentages below:
69.8% of Other AEs (e.g., menstrual changes, herpes simplex, herpes zoster) were either not experienced before or were more severe than those previously experienced.
82.7% of Allergic AEs (rash, itching, difficulty breathing, facial or throat swelling) were either not experienced before or were more severe than those previously experienced.
74.2% of Nerve system AEs (e.g., tingling/itching, Bell's Palsy, vision impairment, memory impairment) were either not experienced before or were more severe than those previously experienced.
59.5% of General AEs (e.g., weakness/tiredness, headache, muscle or joint pain, shaking) were either not experienced before or were more severe than those previously experienced.
43.5% of Local AEs (e.g., pain, limited arm movement, swelling, enlarged lymph nodes) were either not experienced before or were more severe than those previously experienced.
Let's turn our attention to the task of assessing the risk of a serious adverse event during the 21–30 day period following a third dose. The data in the last row of Table 1 above allows us to quantify this risk to some degree.
If we define a serious adverse event as one requiring medical treatment, then we see 72 respondents sought medical treatment for a “General AE.” Thus, the percentage of respondents seeking medical treatment for a General AE is 72/2,049 × 100 ≈ 3.51%. We can’t be sure whether or not the 72 individuals seeking medical treatment for a General AE didn't seek treatment for another AE. However, we can see by adding the totals seeking medical treatment in each category that between 72 and 159 survey respondents sought medical treatment for an AE—that is, between 3.51% and 7.76% of survey respondents experienced a serious adverse event, based on our adopted definition of “serious.” We shouldn't, however, use these percentages to estimate the risk of a serious adverse event.
To get risk-relevant percentages, we need to understand the survey-response rate and make the natural assumption that those experiencing a severe adverse event would be more likely to participate in the survey-interview process. According to information provided on page 8 of the Health Ministry's survey-data report, 2,894 is the natural choice for survey sample size (which is used for the second, response-rate calculation on page 8).
If we assume that all those in the sample of 2,894 who sought adverse-event medical treatment completed a full interview, then we'd conclude that between 2.49% and 5.49% of those in the sample experienced a serious adverse event. Thus, I assert that 3% provides a rough conservative estimate of the risk of a “serious AE” following a 3rd dose of Pfizer's COVID vaccine with “serious” meaning the AE prompted the recipient to seek medical care.
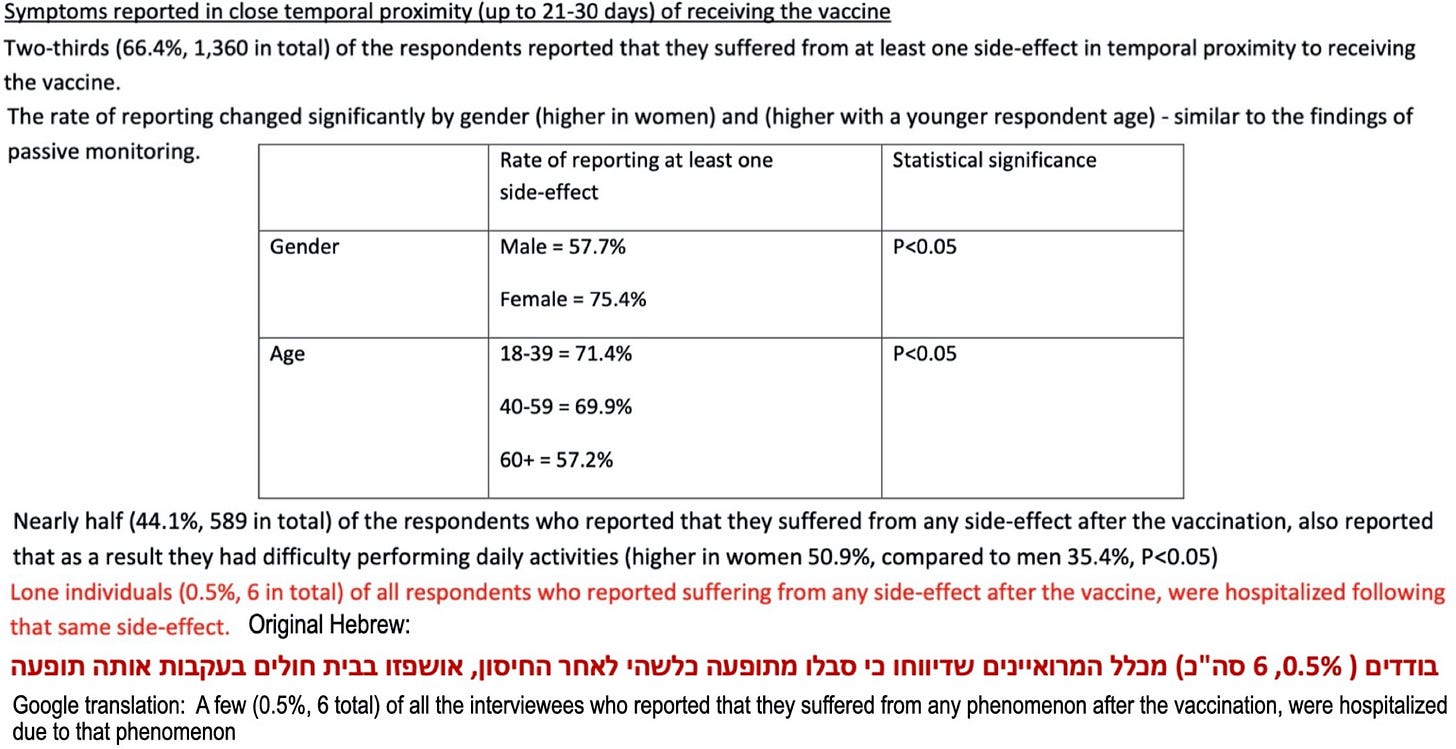
Actually, if considerations of age and gender are included, the risk of a serious adverse event would be higher for females and for younger recipients (say, under age 59) than for males and those 60 and over—see page 10 of the survey, which appears below (with, for the final sentence, the original Hebrew and corresponding Google translation added); data from page 10 will allow us to obtain risk estimates of a serious adverse event using different definitions of “serious.”
Let's consider the risk of an AE that results in having “difficulty performing daily activities” (typically in a short window following vaccination). Such an adverse event wouldn't necessarily be serious, but it would almost certainly be seriously annoying.
Based on demographic data on page 8 of the survey report, 1,005 of the 2,049 survey respondents were women, and, based on the information provided on page 10 above, we assume that 50.9% of these women, 512, did have difficulty performing daily activities following their booster doses. If we assume all those in the sample (2,894) who had difficulty performing daily activities following vaccination did complete the survey process and that half, 1,447, were women, then a rough, conservative, estimate of the risk that a female booster-shot recipient would have trouble performing daily activities after a booster dose is 512/1,447 × 100% ≈ 35.4%, a little higher for those under age 59 and a little lower for those 60 and older. For males, the corresponding risk would be 25.6%.
Let's now consider a more demanding requirement for “serious AE”; namely, the event led to hospitalization. According to the text in red on the survey's page 10 displayed above, six of the survey respondents were hospitalized for a “side effect phenomenon.” Thus, we take 6/2,894 × 100% ≈ 0.21% as an estimate of the risk of hospitalization for a booster-dose-associated adverse event. This is certainly a small risk, but how does it compare to the risk of hospitalization owing to Omicron infection (which is milder than that of previous variants)? To address this question, we use the following estimate:
22,000 young adults would need to be boosted with BNT162b to prevent one Omicron-infection hospitalization over six months.
which is obtained in Section 3.4 of a research paper by Bardosh et al. via adjusting a CDC estimate corresponding to Delta infection to reflect the reduced virulence of Omicron.
We know that, according to the Health Ministry’s survey, hospitalization risk associated with an initial Pfizer-booster-dose is about 0.21%, which means we might expect among 22,000 booster-dose recipients (ages 18 and up) about 0.0021 × 22,000 ≈ 46 hospitalizations for a booster-dose-associated adverse event. It's important to keep in mind that we don't have an age profile for those Israeli citizens reporting hospitalization for a booster-dose-associated adverse event, but we do know that the youngest survey-respondent age-group (18–39) had the highest percentage reporting at least one adverse event. Thus, it’s certainly reasonable to suppose at least 2 of the expected 46 hospitalizations would be of young adults.
Let’s now analyze the health impact of a booster-dose mandate at a college or university having an enrollment of 22,000 students. Let’s call this institution CVMU (COVID-Vaccine-Mandating University), and let’s assume that 55% of CVMU students are females to reflect the reality that the majority of students at U.S. colleges and universities are females. So, CVMU has 12,100 female students and 9,900 male students. Based on the Israeli Health Ministry’s booster-dose survey, we conclude that in an effort to prevent a single Omicron-infection hospitalization among CVMU students during a six-month period, CVMU’s booster mandate will likely result in
at least 2 hospitalizations for a booster-dose-associated adverse-event
approximately 4,283 (≈ 12,100 × 0.354) female students’ having trouble performing daily activities (e.g., attending classes) after a booster dose,
approximately 2,534 (≈ 9,900 × 0.256) male students’ having trouble performing daily activities after a booster dose, and
approximately 660 (= 22,000 × 0.03) students seeking medical care for a booster-dose-associated adverse-event.
The preceding analysis, together with (i) unknown potential long-term risks of repeated boosting, (ii) known risks such as heart injury, and (iii) a dearth of evidence suggesting high vaccination rates on college campuses translate to low infection rates, leads to the conclusion that, beyond any reasonable doubt, mandating booster doses for college students has harms substantially outweighing benefits.
Remark: The Danish Health Authority must believe that the benefits of booster-dosing don't exceed the risks, not only for young adults, but also for most Danes under age 50—see “Why are people aged under 50 not to be re-vaccinated?” in the Q&A section of webpage “Vaccination against covid-19.”
3. Were justifications for COVID-vaccine mandates imposed on college and university students for the academic year 2021-2022 based on science or wishful thinking?
In a message informing the Yale University community that full COVID vaccination would be mandated for all undergraduate, graduate, and professional school students planning to be on campus for the fall 2021 semester, Yale's president and provost provided the following justification for the mandate:
Although the course of the COVID pandemic over the coming months remains uncertain, vaccination is the strongest tool for preventing transmission of the virus. There is abundant evidence of the vaccines’ effectiveness and growing confidence that vaccines will be widely available by early summer.
Read the preceding two sentences carefully. Which of the two justifications offered is more likely to be science based? You guessed correctly---the one for which there is “abundant evidence”: vaccine effectiveness. Describing vaccination as a tool for preventing transmission amounts to wishful thinking; for instance, the FDA's EUA for Pfizer's BNT162b2 states,
At this time, data are not available to make a determination about how long the vaccine will provide protection, nor is there evidence that the vaccine prevents transmission of SARS-CoV-2 from person to person.
Notice that in the justification for Yale's mandate “preventing transmission” precedes “effectiveness.” This is logical, especially if you interpret “effectiveness” to mean effectiveness against severe disease because healthy college students are quite unlikely to experience a severe case of COVID. Thus, the primary justification of Yale's mandate, preventing transmission, was based on wishful thinking, not science. Even the belief that vaccine effectiveness would be high fall '21 for those vaccinated spring '21 amounts to wishful thinking, given the FDA's statement above, “[D]ata are not available to make a determination about how long the vaccine will provide protection.” Perhaps Yale's leaders’ confidence that spring vaccination would yield high fall protection was based on comments made by public-health officials like Anthony Fauci:
Fauci said he was “certain” it [immunity from vaccination] would endure to some extent, as immune system cells called “memory B cells” remained on standby to generate new antibodies to the virus. But for how long was unclear. “We don’t know whether it’s going to be a year, two years, three years, five years, we don’t know,” he said.
Some context: About 7.5 months after the FDA issued its emergency-use authorization for Pfizer's BNT162b2, CNBC reported 23 July 2021 that data from Israel suggested Pfizer's mRNA COVID vaccine to be only 39% effective against the Delta variant, and, on 29 July 2021, the Washington Post reported on the sense of alarm within the CDC that the vaccines the agency had been championing did not appear to be the COVID-vanquishing champions clinical-trial results suggested they might be.
Vaccine mandates being enforced this fall by U.S. colleges and universities are based solely on wishful thinking. During the 2021-2022 academic year, many colleges and universities had serious COVID outbreaks despite having extremely high vaccination rates on campus. Cornell University's spring 2022 outbreak provides an excellent example. Here's the beginning of a story titled “New Cornell Outbreak: Nearly 3% of Undergrads Test Positive in 3 Days” posted at the website of a New York NBC affiliate 26 March 2022:
Cases of COVID-19 have surged at Cornell University in recent days, creating a sense of déjà vu for a school that sounded the alarm in early winter when an omicron outbreak closed facilities and moved finals online. The last three of days of recorded data on the university's website show a measurable spike in positive tests among undergraduate students, whose population boasts a 97% vaccination rate. In that time period, at least 503 cases of the virus have been detected among all students, staff and faculty, more than 80% of which are from the undergrad body. The more than 400 students who tested positive between Tuesday and Thursday this week account for nearly 3% of all undergraduate students (2.68%, to be precise).
It's worth noting that Cornell University had a booster mandate in place for its spring 2022 term, and we see in Cornell University's discussion of the mandate (21 December 2021) more evidence of unjustified wishful thinking concerning the effectiveness of COVID vaccines (in this case boosters): “As Dr. Anthony Fauci M.D. ’66 noted just last week, recent studies indicate that Moderna and Pfizer boosters are likely to offer substantial protection against the highly-transmissible Omicron variant.” The boosters did not offer substantial protection—a little over 3 months after announcing this mandate, Cornell had the major COVID outbreak on campus described in the NBC story above.
College leaders enforcing mandates fall 2022 have no reason to expect students vaccinated August 2022 will have significant protection from infection by February 2023. Consider, e.g.,data provided by Table 3a “Consensus vaccine effectiveness estimates” from the 4 August 2022 UK Health Surveillance Report:
Dose 2 of BNT162b2, provides after 6 months, 20% (10-30) effectiveness in preventing all infection and 15% (10-15) against symptomatic infection.
A booster dose of BNT162b2, provides after 6 months, 0% (0-10) effectiveness in preventing all infection and 10% (0-20) against symptomatic infection.
Dose 2 of mRNA-1273, provides after 6 months, 30% (10-50) effectiveness in preventing all infection and 15% (10-20) against symptomatic infection.
A booster dose of mRNA-1273, provides after 6 months, 0% (0-10) effectiveness in preventing all infection and 10% (0-20) against symptomatic infection.
Note in particular that in the long run those boosted have a greater risk of infection than those having only the primary series. Also note that if you believe the vaccinated have a greater chance of experiencing a mild case or asymptomatic case of COVID-19, then you should acknowledge that the vaccinated are more likely to transmit COVID to others because they are more likely to be out and about rather than at home in bed not feeling so well.
Given
high vaccination rates don't protect campus communities from COVID-19 outbreaks, partially owing to dramatically waning vaccine effectiveness over time,
infection in healthy students is, with near certainty, not going to be severe (fall 2020, so many colleges and universities were reporting large numbers of COVID cases among students with 0 associated hospitalizations, that fact-check articles were generated to emphasize that there were actually some hospitalizations occurring),
vaccination does have potentially serious, known life-changing or life-ending risks, as well as unknown long-term risks.
Part 2 will be released on December 8, 2022
Paul Bourbon is a Professor of Mathematics, General Faculty, University of Virginia (Retired); Formerly, Cincinnati Professor of Mathematics at Washington & Lee University



Push back now. Push back hard. In BC Canada they passed legislation to jail health practionners for up to 2 years for covid "misinformation" and to vaccinate by Force a health practitioner who refuses. Etc
Etc. Bill 36
Loving these faculty letters of support. Keep them coming!! We need like 1,000,000 more of them to right this ship....