Last week, I raised and commented on the following three questions relating to the logic of mandating COVID vaccination:
1. To what extent, if any, have those mandating COVID vaccines considered potential long-term risks?
2. Do risks of short-term side-effects diminish with each dose of a COVID vaccine?
3. Were justifications for COVID-vaccine mandates imposed on college and university students for the academic year 2021-2022 based on science or wishful thinking?
Descriptions of how the press has failed to report news objectively during the coronavirus pandemic provide a unifying theme in the discussion of my final three questions concerning the logic of COVID-vaccine mandates:
4. Have those who have mandated COVID vaccination been influenced by vaccine misinformation?
5. Is it logical to mandate use of products produced by untrustworthy companies like Pfizer?
6. Is forcing one’s faith on others logical?
Because thorough documentation is required for some of the examples of misinformation for question 4 as well as some of the examples of Pfizer’s untrustworthiness for question 5, part 2 of my essay is rather long. Thus, to read the entire post, you’ll need to choose either “Open in app · Open in browser” immediately above the No College Mandates banner at the top of this message, or “View entire message” when you reach the end of the emailed portion.
4. Have those who have mandated COVID vaccination been influenced by vaccine misinformation?
News is what someone wants suppressed. Everything else is advertising. The power is to set the agenda. What we print and what we don't print matter a lot.
- Katharine Graham, CEO of The Washington Post Company, 1972–1991
I wonder how different the U.S.'s response to the coronavirus pandemic would have been were Katharine Graham still leading the Washington Post. The current leadership of the Post should offer an apology similar to the one titled “We failed,” appearing 7 January 2022 in Ekstra Bladet, a Danish tabloid newspaper founded in 1904. Here are some excerpts from the apology, which was written by journalist Brian Weichardt:
[W]e—the press—must also take stock of our own efforts. And we have failed.
WE HAVE NOT been vigilant enough at the garden gate when the authorities had to demand an answer to what it actually meant that people are hospitalized with corona and not because of corona. Because it makes a difference. A big difference. Precisely, the official admission figures have been shown to be 27 percent higher than the real figure for how many are in hospital, solely because they have corona. We only know that now. …
ANOTHER example: Vaccines are consistently referred to as our “superweapon”. And our hospitals are called “super hospitals”. Nevertheless, these super-hospitals are obviously under maximum pressure, even though almost the entire population is armed with a super-weapon. …
IN OTHER WORDS, THERE IS something here that does not deserve the term “super”. … But the communication of those in power to the population in any case does not deserve the designation “super”. On the contrary.
I provide below some examples of misinformation that may have influenced (or reassured) those responsible for, or supportive of, vaccine mandates.
Vaccine-effectiveness misinformation
You’re not going to—you're not going to get COVID if you have these vaccinations
- President Joseph Biden, 21 July 2021
A little over a week before President Biden offered the preceding misinformation, Israel National News reported 13 July 2021, “Nearly 40% of new COVID patients were vaccinated—compared to just 1% who had been infected previously.” These percentages are based on 7,700 new cases of the virus detected “during the most recent wave starting in May” with only 72 of the confirmed cases among the previously infected but more than 3,000 among the fully vaccinated.
Vaccine-approval misinformation
The State Department’s Global Engagement Center has found that Russia and China have promoted their own vaccines through messaging that undermines Western origin vaccine development programs. … This is what they’re—what the information—some of this misinformation is doing—and misleads the public by falsely alleging that mRNA vaccines are untested and, thus, risky, even though many of them are approved and have gone through the gold standard of the FDA approval process.
- White House Press Secretary Jen Psaki, 16 July 2021
The FDA approved the first COVID vaccine (Pfizer's) on 23 August 2021, over a month after the preceding misinformation was provided by Jen Psaki. Pfizer's vaccine was granted an Emergency Use Authorization on 11 December 2020 and Moderna's, on 18 December 2020. Let's modify the conclusion of Ms. Psaki's statement above, so that it becomes truthful:
… misleads the public by falsely alleging that mRNA vaccines are untested and, thus, risky, even though Pfizer's and Moderna's vaccines have been granted an Emergency Use Authorization by the FDA.
The truth would not inspire in those listening the confidence in the vaccines that Ms. Psaki desired to impart. Thus, she ignored the truth.
COVID Hospitalization-Rate Misinformation
Just as in Denmark, “authorities” in the U.S. have misrepresented COVID-hospitalization statistics. Here are the first two paragraphs of a Newsweek article appearing 11 January 2022.
CNN anchor Jake Tapper has criticized as “misleading” the admission by the head of the Centers for Disease Control (CDC) and Prevention that it counted COVID patients who had been admitted to hospital for something else.
Tapper was reacting to comments CDC director Rochelle Walensky made on Fox News on Sunday that “up to 40 percent” of patients had been admitted to hospitals with another medical emergency but had been later detected as having COVID.
Remark: The headline of another Newsweek article appearing 11 January 2022 reports “Almost Half of Ontario COVID Hospitalizations Are ‘Incidental,’ Admitted for Other Reasons.”
Vaccine-effectiveness misinformation
An FDA press release, 1/31/2022, regarding the approval of Moderna's Spikevax, includes the following:
The public can be assured that Spikevax meets the FDA’s high standards for safety, effectiveness and manufacturing quality required of any vaccine approved for use in the United States,” said Acting FDA Commissioner Janet Woodcock, M.D.
In June of 2020 the FDA's “high standards” for COVID-vaccine efficacy are described as follows:
To ensure that a widely deployed COVID-19 vaccine is effective, the primary efficacy endpoint point estimate for a placebo-controlled efficacy trial should be at least 50%, and the statistical success criterion should be that the lower bound of the appropriately alpha-adjusted confidence interval around the primary efficacy endpoint point estimate is > 30%.
While it's true that back in December of 2020, Moderna's phrase III clinical trial suggested, after about 2 months of data collection beyond the 2nd dose, an efficacy rate of 94.1% for its mRNA-1273 vaccine (later called Spikevax), we now have extensive real-world effectiveness data. This data convincingly shows that against the Omicron variant, Moderna's Spikevax, does not meet the “high standards” for effectiveness the FDA itself set June of 2020.
Consider the following plot from a Danish cohort study, posted 23 December 2021:
The plot suggests that against the dominant Omicron variant, Moderna's Spikevax is less than 50% effective 1–31 days after full vaccination, and protection drops significantly after two months to a level near 0 and becomes negative after about three months. Does this plot reflect the FDA's “high standards” for effectiveness?
Similarly, odds-ratio data from an article appearing in the Journal of the American Medical Association (see Figure 2c) yields effectiveness data (calculated as 1 minus the odds ratio) similar to that of the Danish study: it suggests that against the dominant Omicron variant, a two-dose series Moderna's Spikevax has maximum effectiveness of less than 50% shortly after full vaccination and drops to under 10% roughly six months after full vaccination.
In light of Spikevax's very limited effectiveness against the dominant Omicron variant, what is the logic behind the FDA's approval of the vaccine for individuals 18 years of age and older? This question is answered in the FDA's Spikevax-approval press release: “The data used for the analyses [of Spikevax's efficacy] were accrued before the Omicron variant emerged.” Is the FDA obligated to approve vaccines based on outdated efficacy data, which, in this case, is essentially irrelevant to those who will receive two doses of the fully-approved vaccine?
Misinformation concerning COVID-19's infection-survival rate
On 26 July 2022, NPR aired an interview “Dr. Fauci on federal response to monkeypox and COVID.” Shortly after the interview started, host Juana Summers said,
We should note here, as we have this conversation, monkeypox is nowhere near as deadly as COVID. Ninety-nine percent of people who get monkeypox can expect to survive.
I sent the preceding quotation (along with other examples of NPR misinformation) to some friends and family. To justify my claim that Ms. Summers was spreading misinformation, I provided the following two paragraphs.
Here’s a passage from an AP fact-check article dated 23 July 2021:
THE FACTS: As of July 23, there were more than 34.3 million known cases of COVID-19 in the United States and 610,370 deaths, according to data from Johns Hopkins University. That means the case fatality ratio—or the portion of known cases that result in death in the country—is 1.8%. In other words, on average, 98.2% of known COVID-19 patients in the U.S. survive. Because the true number of infections is much larger than just the documented cases, the actual survival rate of all COVID-19 infections is even higher than 98.2%.
The CDC estimates that 1 in 4 (95% CI: 3.4, 4.7) COVID-19 infections were reported during the period February 2020–September 2021 in the U.S. In other words, during the period February 2020–September 2021, to get a rough estimate of the number of persons in the U.S. having been infected by SARS-CoV-2 by a certain date, you'd multiply the corresponding number of reported cases by 4. Also, on the same webpage, the CDC asserts that COVID deaths are under-reported: to obtain a good estimate of actual number of COVID deaths from the reported number, we should multiply by 1.32. Using these CDC estimates and the AP fact-check data above, we obtain an infection fatality rate of 1.32/4 × 1.8% = 0.594%, corresponding to an infection-survival rate of 99.41% (as of 23 July 2021).
For the milder omicron variant, the case fatality rate is significantly lower. Consider, e.g., a Johns Hopkins report“Comparing cases, deaths, and hospitalizations indicates Omicron less deadly” dated 7 March 2022: “During the Omicron wave, mortality decreased significantly, with deaths now representing less than 0.5% of reported cases.” Thus, the Omicron case survival rate is over 99.5%, and the infection survival rate is over (100 - 1.32/4 × 0.5)% = 99.835%.
One of the recipients of my message to friends and family about NPR misinformation, responded as follows:
I assume the 99% survival rate with monkeypox is without vaccination, whereas the 99% covid survival rate you cited is with a majority of US citizens being vaccinated. So perhaps the reporter was intending to compare with the fatality rate for unvaccinated covid patients.
What follows is a slightly modified version of my response:
I’m surprised you believe that vaccination might have a significant impact on COVID-survival rates. Results of COVID vaccine clinical trials certainly don’t support this belief, but let’s just look at the data for now. According to the CDC, as of Dec 30, 2020, well before any members of the general public were 14 days past their 2nd COVID shot, there were 357,407 deaths with COVID in the USA and 19,901,933 COVID cases.
This gives a case fatality rate of 357407/19901933 ≈ 0.018; i.e., 1.8%, and thus a case-survival rate of 98.2%. This is exactly the same as the AP fact-check survival rate of 23 July 2021. The vaccines had no impact on case-survival rate. Of course, as we noted above, the infection-survival rate is much higher---about 99.41% according to the CDC case-to-infection multiplier of 4 and death multiplier of 1.32. Also, because the vast majority of COVID deaths occur in the elderly, the infection-survival rate for the young would be higher than 99.41%.
There was no reason to expect vaccines to have a significant impact on survival rates. The reason you expected an impact is that news outlets like NPR reported vaccine efficacy (based on clinical trials) only in terms of relative-risk reduction of symptomatic infection (95% Pfizer; 94.1% Moderna). However, the absolute risk reduction, computed from trial data, was very small: 0.84% for Pfizer and 1.2% for Moderna. In other words, in, say, Pfizer’s trial, those receiving the vaccine were only 0.84% percent less likely to experience symptomatic infection with COVID than those in the placebo group. The mortality risk reduction would be significantly smaller—in fact, there were no deaths caused by COVID in either the treatment group or the placebo group in the portion of Pfizer’s trial from which the 95% relative-risk reduction derives—see the NEJM article in which Pfizer reported on results of its phase III trial.
Back when the NIH, FDA, and CDC were not simply marketing agencies for the pharmaceutical industry, the NIH published a book titled Smart Health Choices. Chapter 18 is titled “Relative risk, relative and absolute risk reduction, number needed to treat and confidence intervals.” The third paragraph begins, “Absolute risk reduction (ARR)---also called risk difference (RD)---is the most useful way of presenting research results to help your decision-making.” So why weren’t news outlets, as well as the FDA and CDC, reporting absolute risk reduction for COVID vaccines (along with relative-risk reduction) to help with “your decision making”?
More vaccine-effectiveness misinformation
On Sunday 19 June 2022, NPR aired a segment titled, “White House COVID-19 response coordinator outlines timeline for vaccinating children,” during which a reporter interviewed Ashish Jha, the White House Covid-19 response coordinator. The piece begins, “The wait is over. Parents can breathe a sigh of relief; COVID-19 vaccines are now recommended for children as young as six months.”
The first request the interviewer makes of Jha is “Can you give us a quick overview? There are two options for age groups and there are some differences; what do parents need to know?” Jha responds,
There are two options Moderna and Pfizer. People will not be surprised to hear that. What parents need to know is both of them are extremely safe and both of them were found by the independent scientific experts at the FDA and CDC to be highly effective. So, the good news is that it's nice to have choices and they both work really well.
Jha's assertion that the vaccines are “extremely safe” is certainly questionable—the vaccine doses administered to young children are simply smaller doses of the same vaccine given to adults. Therefore, the potential risks of vaccination described in, say, the FDA's package insert” information for Pfizer’s COVID vaccine apply to young children.
What is clearly misinformation is Jha's conveying that vaccines for toddlers are “highly effective.”
I've already mentioned that in June of 2020 the FDA's standards for COVID-vaccine efficacy are described as follows:
To ensure that a widely deployed COVID-19 vaccine is effective, the primary efficacy endpoint point estimate for a placebo-controlled efficacy trial should be at least 50%, and the statistical success criterion should be that the lower bound of he appropriately alpha-adjusted confidence interval around the primary efficacy endpoint point estimate is > 30%.
Let's see how the “highly effective” toddler vaccines measure up to the preceding FDA standard.
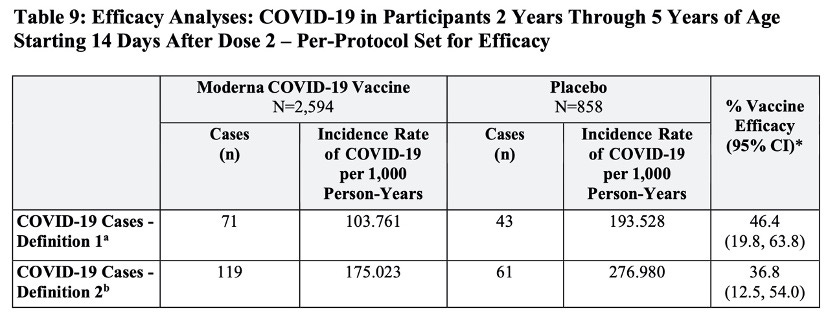
Here are Tables 8 and 9, p. 34 from Moderna's “Toddler Trial” report:
Note that the 50% efficacy standard is met only for those of ages 6 through 23 months (with case definition b) and that the 30% standard for the lower bound of the 95% confidence interval is quite far from being met in all four cases. I imagine that a top reporter for Katharine Graham's Washington Post, upon viewing the preceding data, would ask the following questions to Jha:
Why has the FDA relaxed its efficacy standards for age groups at such a low risk of harm from coronavirus infection?
Is there is a coronavirus emergency at this point, especially for toddlers?
Regarding the case definitions appearing in the footnotes to Table 8, don't you agree that many children in the age range 6 through 23 months will not be able to communicate that they are having chills, a headache, sore throat, or a loss of smell/taste? Doesn't this call into to question the scientific integrity of Moderna's trial-design for this age group?
Does this data justify a claim that the vaccines are “highly effective” for children 6 months to 5 years?
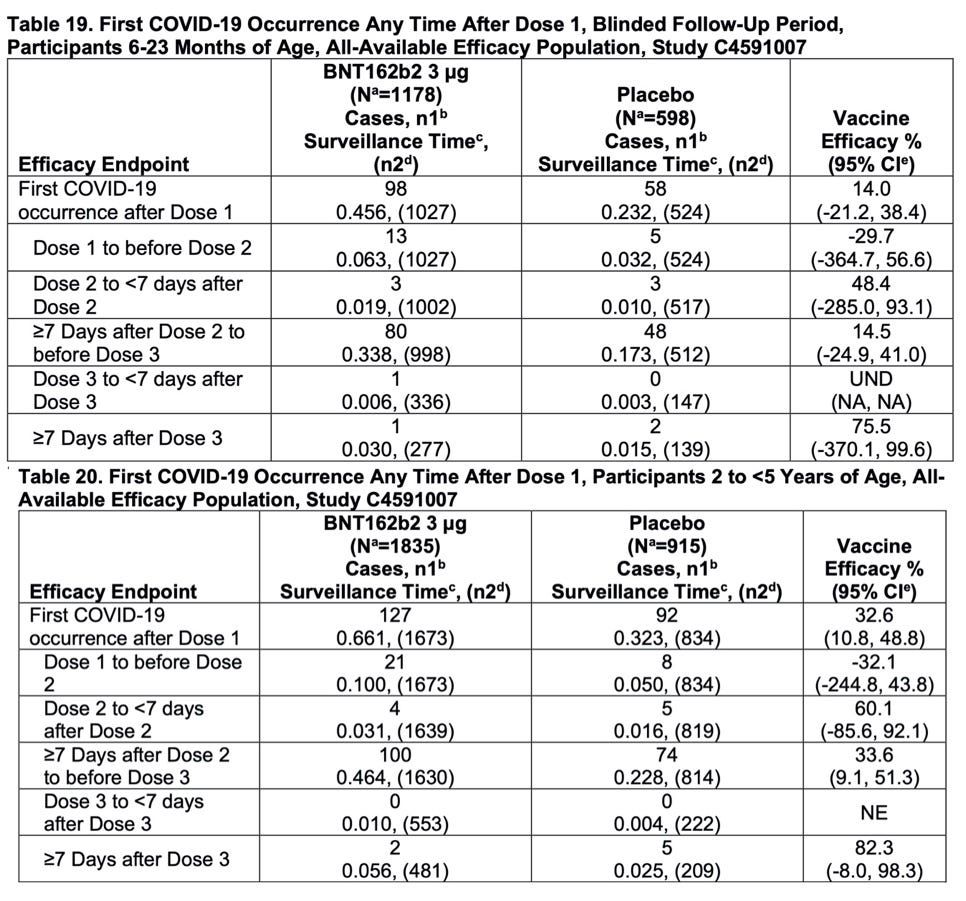
Let's take a look at Pfizer's efficacy data (Tables 19 and 20 on page 39):
Note that final efficacy estimates are based on a total of only 10 cases (combining cases in both tables). Also note the lower bounds of the confidence intervals for efficacy of the FDA emergency-used authorized 3-dose series are negative, when they should be at least 30% to meet the FDA's standard for COVID vaccines. Pfizer had wished to provide efficacy data based on a total of at least 21 cases, but the company was rushed by the FDA and the Biden administration to seek an EUA before that many cases occurred in their trial.
There's an even more important point to make regarding Pfizer's efficacy data. Restricting efficacy/effectiveness analysis for Pfizer's vaccination program to the period 7 or more days after the third dose does not give a full picture of vaccine effectiveness. Shouldn't all cases occurring after the first dose be considered as part of the efficacy/effectiveness analysis of the three-dose vaccination series? Looking at the top row of Tables 19 and 20, we see that the overall efficacy of Pfizer's 3-dose vaccination program for children 6 months through 23 months is only 14.0% and that for children between 2 years and 5 years is only 32.6%.
Thus, on 19 June 2022, NPR allowed White House COVID-19 response coordinator Ashish Jha to make, unchallenged, the false claim that Moderna's and Pfizer's COVID19 vaccines for young children are “highly effective.”
Vaccine-safety misinformation
According to CNN, Ashish Jha, the White House Covid-19 response coordinator, commented on COVID-vaccine safety for toddlers, as follows, on CBS on Monday, June 27:
It's very similar to the side effects we've seen for older kids or for adults. About 24 hours of some kids, you know, they kind of don't feel as well, they feel tired, they don't have the same appetite. But thankfully, there have not been any serious side effects of these vaccines.
Jha's assertion that “there have not been any serious side effects of these vaccines” is false. E.g., two serious side effects were documented in clinical trials for toddlers. According to an article appearing in Nature:
The firms [Moderna and Pfizer] disclosed that serious adverse reactions related to the vaccine had occurred, but were rare. Moderna, based in Cambridge, Massachusetts, reported that one child who received its vaccine had a seizure triggered by a high fever (see ‘Moderna paediatric-trial results, at a glance’), and Pfizer, based in New York City, reported one case of fever and calf pain that might have been linked to vaccination (see ‘Pfizer paediatric-trial results, at a glance’).
Also, I wonder if Jha is unfamiliar with the “package insert” information (mentioned earlier) concerning the risks associated with Pfizer’s COVID vaccine (Comirnaty), which makes it clear that there are serious side effects associated with “these vaccines.” Consider, for example,
Section 6.2 Postmarketing Experience. The following adverse reactions have been identified during postmarketing use of COMIRNATY, including under Emergency Use Authorization. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Gastrointestinal Disorders: diarrhea, vomiting
Immune System Disorders: severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema)
Musculoskeletal and Connective Tissue Disorders: pain in extremity (arm)
In addition, it appears that Jha has not read the FDA's press release announcing its full approval of Moderna's Spikevax, which includes the following:
Additionally, the FDA conducted a rigorous evaluation of the post-authorization safety surveillance data pertaining to myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of tissue surrounding the heart) following vaccination with the Moderna COVID-19 Vaccine and has determined that the data demonstrate increased risks particularly within seven days following the second dose, with the observed risk highest in males 18 through 24 years of age. Available data from short-term follow-up suggest that most individuals have had resolution of symptoms. However, some individuals required intensive care support. Information is not yet available about potential long-term health outcomes. The Spikevax Prescribing Information includes a warning about these risks.
“These vaccines” have also been linked to deaths: e.g., Simone Scott, 19, Drene Keyes, 58, New-Zealand man, 26, Gregory Michael, 56, and George Watts, Jr., 24.
5. Is it logical to mandate use of products produced by untrustworthy companies like Pfizer?
There is clear and convincing evidence, accumulating before, during, and after Pfizer's development of its COVID-19 vaccine, that Pfizer is not a company worthy of anyone's trust (except possibly its shareholders). Let's examine some of this evidence, starting with an analysis of Pfizer's response to an Israeli Health Ministry report issued Spring 2021.
Early Warnings of Myocarditis as an Adverse Reaction to Vaccination
A Times of Israel article, appearing 23 April 2021, describes an Israeli Health Ministry intermediate report raising concerns about a possible class of adverse reactions to Pfizer's COVID vaccine: “There could be a link between the second shot and several dozen cases of myocarditis, an inflammation of the heart muscle, particularly in men under 30.” An excerpt from the report states, “The findings were presented to the Pfizer company who replied that they had not had similar reports in the rest of the world and would examine the data.''
Note the date of the Times article: 23 April 2021. The article states, “The concerns come from an intermediate report that was presented to ministry heads and to Pfizer in recent weeks.” Being generous, let's assume “recent weeks” represents the period 23 March 2021–23 April 2021. I'll prove below that there were “similar reports in the rest of the world” well before 23 March 2021. One source of such reports is the CDC's VAERS (Vaccine Adverse Events Reporting System) database.
If you perform an appropriate VAERS search, choosing “COVID19 (COVID19 VACCINE)” for “Vaccine Products”; “PFIZER-BIONTECH” for “Vaccine Manufacturer,” and “myocarditis” as an adverse-event keyword, then VAERS will return, for January 2021, 9 total events with “myocarditis”' appearing in the adverse-event description, and 28 such events for February 2021. Here's one of these adverse-event descriptions:
VAERS ID: 0998532-1. Received 2/3/21. The patient was admitted to the hospital on 24 January with chest pain, elevated troponin, and EKG changes in the setting of a couple days of chills, sweats and malaise after receiving the covid vaccine. Initially there was concern for acute MI and he was taken for a coronary angiogram. The angiogram was clean and showed no obstructive disease. He had a cardiac MRI which showed findings consistent with myocarditis. He was treated with supportive care and eventually was discharged. He was worked up for other causes of myocarditis with none to be found.
Thus, by March 1st, 2021, there were at least 37 reports in the CDC's VAERS database, relating to Pfizer's COVID vaccine, with “myocarditis” mentioned in the adverse-event description. Moreover, Pfizer itself had received reports of post-vaccination myocarditis cases before March 1st. This is known because the FDA was forced by a court order to begin releasing documents it received from Pfizer relating to its BNT162b COVID vaccine.
Information in documents released on March 1st, 2022 includes a nine-page list of adverse-event reports received by Pfizer during the period 1 December 2020 to 28 February 2021. You may read about this adverse-event list in a Reuters fact-check article. According to this article, “Examples of such events are difficult breathing, déjà vu and myocardial infarction, as well as 1,223 events with a fatal outcome (here).” If you follow the link “here” and proceed to the table on page 20, you'll see the list of adverse events includes myocarditis. Therefore, Pfizer was aware of reports of myocarditis following vaccination when it responded to Israel's Health Ministry in late March or early April: “[T]hey had not had similar reports in the rest of the world and would examine the data.”
Perhaps Pfizer representatives communicating with Israel's Health Ministry were not aware of reports of post-vaccination myocarditis the company had received or those that had appeared in VAERS—even so, wouldn't a trustworthy company have well-informed representatives that do not communicate false information to public-health agencies (for example, the U.S. CDC and FDA or Israel's Ministry of Health)?
The CDC now has a webpage, titled “Myocarditis and Pericarditis After mRNA COVID-19 Vaccination” that includes the following statement:
Most patients with myocarditis or pericarditis who received care responded well to medicine and rest and felt better quickly.
The CDC's failure to monitor its own adverse-event reporting system and Pfizer's failure to act on its own internal reports (and seek more evidence of a vaccine-myocarditis link by, e.g., consulting with the CDC about VAERS reports) meant that the world was not warned of the link between COVID vaccination and myocarditis/pericarditis until late April of 2021. In particular, doctors who might have provided the care, the importance of which CDC now emphasizes, to young persons suffering from post-vaccine myocarditis were also not warned.
Surely, Moderna had reports of myocarditis as well and failed to consult with the CDC. This is gross negligence and it caused harm. For example, Northwestern University student Simone Scott died 11 June 2021 about one month after receiving her second dose of Moderna's mRNA-1273—see the article “Northwestern University student appears to have died from heart inflammation linked to COVID vaccine” in Northwestern's newspaper The College Fix.
Independent journalist Alex Berenson reports,
Unfortunately, doctors appear to have repeatedly missed signals as Simone’s condition slowly worsened in the two weeks following her second shot—before she abruptly crashed. … She suffered serious short-term side effects after her first Moderna dose on April 3 and never fully recovered, her parents said.
Had Ms. Scott's doctors known of the vaccine-myocarditis link, perhaps Simone would have received appropriate care and “responded well.” Should the CDC, Moderna, and Pfizer be free from liability given their gross negligence and the harm it caused?
Scientific Shortcomings of Adult Clinical Trials for mRNA COVID Vaccines
Pfizer was responsible for the design and conduct of the trial, data collection, data analysis, data interpretation, and the writing of the manuscript.
- From Pfizer's NEJM article describing results of its phase III trial for BNT162b2
The system of drug testing and approval employed in the U.S. is fundamentally flawed. We have vaccine manufacturers testing their own products in clinical trials they manage. These manufacturers have a clear conflict of interest—they want to develop and sell vaccines to make a profit. For regulatory-agency approval as well as marketing, they have every motivation to exaggerate their vaccine's efficacy and safety.
Were the manufacturers liable for product defects, there might be some reason to trust the results of the clinical trials they conduct. Lack of liability, poor regulatory oversight from the FDA (evidence forthcoming), and obvious conflicts of interest of those managing clinical trials means, logically speaking, the public has reason to be skeptical that “the science” was carefully followed in the course of any vaccine clinical trial.
Furthermore, keep in mind that those in the FDA who set standards and goals for clinical trials are allowed to move on to positions in the pharmaceutical companies whose products they recently evaluated for the public. For instance, former FDA commissioner Scott Gottlieb is now a member of Pfizer's board of directors. According to CNBC article questioning the propriety of his joining the board “just two months after stepping down from the agency that regulates the drugmaker,” Pfizer’s board members were paid a minimum of $335,000 in stock and cash in 2018.
Let's examine some evidence suggesting that “the science” was not well followed in the (adult) clinical trials for mRNA COVID vaccines.
Two pharmaceutical companies developed mRNA COVID vaccines that eventually received EUAs for administration in the United States: Pfizer and Moderna. The COVID-vaccine adult clinical trials for both these companies had the same “primary end point”:
prevention of symptomatic, laboratory-test confirmed Covid-19 illness.
A clear scientific shortcoming of both trials is that, as Pfizer states in its NEJM paper describing its clinical-trial results, “These data do not address whether vaccination prevents asymptomatic infection,” while Moderna states, “These data were not sufficient to assess asymptomatic infection ….” Why is this lack of data on asymptomatic cases a scientific shortcoming? Because asymptomatic transmission of the SARS-CoV-2 virus is considered to be a significant factor in its spread—in fact, asymptomatic transmission was a major justification of lockdown policies. Not gathering data (or sufficient data) on asymptomatic cases means that Pfizer's and Moderna's trial results do not speak to their vaccines’ ability to limit the spread of COVID. Indeed, in a BMJ (British Medical Journal) featured editorial, the chief medical officer at Moderna Tal Zaks is quoted as follows:
“Our trial will not demonstrate prevention of transmission,” Zaks said, “because in order to do that you have to swab people twice a week for very long periods, and that becomes operationally untenable.''
Thus statements, like the one below Anthony Fauci made on CBS's Face the Nation Sunday, 16 May 2021, are not supported by science:
When you get vaccinated, you not only protect your own health and that of the family but also you contribute to the community health by preventing the spread of the virus throughout the community. In other words, you become a dead end to the virus. And when there are a lot of dead ends around, the virus is not going to go anywhere. And that's when you get a point that you have a markedly diminished rate of infection in the community.
Moreover, at the time (May 16th, 2021) Fauci made the preceding comment, he should have already reviewed a preliminary analysis of safety-and-efficacy data for Pfizer's vaccine, collected through March 13, 2021. This data, not reported to the public until 28 July 2021, reveals waning protection from vaccination:
From its peak post-dose 2, observed VE [vaccine efficacy] declined. From 7 days to < 2 months post-dose 2, VE was 96.2% (95% CI [93.3-98.1]); from 2 months to <4 months, VE was 90.1% (95% CI [86.6-92.9]); and from 4 months to the data cut-off, VE was 83.7% (95% CI [74.7-89.9]).
A peer-reviewed study illustrating that Fauci's assertion is not valid, titled “Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States,” concludes,
At the country-level, there appears to be no discernible relationship between percentage of population fully vaccinated and new COVID-19 cases in the last 7 days. … In fact, the trend line suggests a marginally positive association such that countries with higher percentage of population fully vaccinated have higher COVID-19 cases per 1 million people.
There is an even greater scientific shortcoming of the COVID vaccine clinical trials—the early elimination of their control groups. BMJ editor Peter Doshi, in his Scientific American article “How to Expand Access to COVID Vaccines without Compromising the Science” (12/17/20) describes this shortcoming as follows:
The colder months have arrived, and COVID-19 infections and deaths are rising once again—but Americans are breathing a sigh of relief nonetheless, as the first authorized vaccine is finally rolling out. Perhaps surprisingly, though, that will actually make it increasingly difficult for public health officials to judge whether any of the vaccines will work well enough to tame the pandemic.
That's because the emergency use authorization (EUA) the Food and Drug Administration (FDA) recently granted to Pfizer will begin to undermine the scientific integrity of the double-blinded clinical trial the company—and other companies—have been conducting, before statistically valid information can be gathered on how effectively the vaccines prevent hospitalizations, intensive care admissions or deaths. All that effort to recruit tens of thousands of volunteers and randomly give them either the vaccine or a placebo will get compromised as people in the placebo half are offered the opportunity to “cross over” and get the vaccine itself.
Doshi describes an “expanded access” alternative to the FDA's EUA that would have given at-risk individuals access to vaccines without compromising the science of the clinical trials. The FDA's EUAs effectively ended, prematurely, COVID vaccine clinical trials.
Perhaps the most important information loss, resulting from premature elimination of clinical-trial control groups, is that of all-cause mortality comparisons between treatment (vaccinated) groups and control (placebo) groups over an extended period of time.
As part of its application to the FDA for full licensure of its COVID vaccine, Pfizer did provide all-cause mortality data for its abbreviated clinical trial, On page 23 of one report dated 11/8/2021, we find
From Dose 1 through the March 13, 2021 data cutoff date, there were a total of 38 deaths, 21 in the COMIRNATY [Treatment] group and 17 in the placebo group. None of the deaths were considered related to vaccination.
An earlier preprint, appearing 28 July 2021 and providing data from dose 1 to unblinding, reports 15 deaths in the treatment group and 14 in the placebo group. On page 12 of a supplement to the report, immediate causes of deaths are tabulated:
As you can see, highlighted in yellow, there were 3 COVID-caused deaths, with 1 in the treatment group and 2 in the placebo group. However, highlighted in pink, we see there were 11 deaths owing to heart problems with 7 in the treatment group and 4 in the placebo group.
There is certainly not enough data here for speculations such as “an unvaccinated person is twice as likely to die from COVID as a vaccinated one” or “a vaccinated person is nearly twice as likely to die from a heart-related issue than an unvaccinated one.” However, who would argue that having data like this for a longer period of time wouldn't provide valuable, and potentially life-saving information, relating to the risks and benefits of vaccination?
There are other reasons to question the science of the mRNA-vaccine clinical trials. On 2 November 2021, a BMJ investigation, titled “Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial,” reports,
A regional director [the whistle blower] who was employed at the research organization Ventavia Research Group [hired by Pfizer to manage some clinical-trial sites] has told the BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial.
We do not know the extent to which the whistle-blower's allegations are true because the FDA failed to conduct a site investigation based on this whistle-blower's complaint. According to a more recent BMJ investigation, published 16 November 2022,
[E]xperts have criticised the FDA’s oversight of clinical trials, describing it as “grossly inadequate.” They say the problem, which predated covid-19, is not limited to a lack of inspections but also includes failing to notify the public or scientific journals when violations are identified—effectively keeping scientific misconduct from the medical establishment.
Is the FDA providing adequate regulatory oversight of vaccine clinical trials? Additional convincing evidence that the answer to this question is no relates to an adverse reaction to vaccination, discussed in the next section, of a participant in Pfizer's phase III clinical trial for 12–15 year-olds
Criminal Conduct
I've already given three reasons to distrust Pfizer: the company provided false information to Israel's Health Ministry, the FDA does not provide adequate regulatory oversight of Pfizer's research, and Pfizer has liability protection for its COVID vaccine.
There are other reasons not to trust Pfizer. For example, a September 2009 Justice-Department press release begins,
American pharmaceutical giant Pfizer Inc. and its subsidiary Pharmacia & Upjohn Company Inc. (hereinafter together “Pfizer”) have agreed to pay $2.3 billion, the largest health care fraud settlement in the history of the Department of Justice, to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products, the Justice Department announced today.
Currently, Pfizer should be under criminal investigation for misclassifying an adverse reaction to a dose of its COVID vaccine administered to a participant in its phase III clinical trial for children 12 to 15 years of age.
The initial “crime” of this particular clinical trial is that it involved only 2260 children, 1131 of whom were in the treatment (vaccinated) group. Pfizer's corresponding trial for adults, involving 43,548 participants, was nearly 20 times larger. Given that the potential benefits for children of COVID vaccination are significantly lower than those for adults (particularly the elderly), the principal aim of this clinical trial for children should have been to conduct a thorough assessment of vaccine safety; yet the trial was too small to achieve that end. The peer-reviewed paper “Why are we vaccinating children against COVID-19?” characterizes COVID-vaccine trials conducted for adolescents/children as follows:
Clinical trials for these inoculations were very short-term (a few months), had samples not representative of the total population, and for adolescents/children, had poor predictive power because of their small size. Further, the clinical trials did not address changes in biomarkers that could serve as early warning indicators of elevated predisposition to serious diseases. Most importantly, the clinical trials did not address long-term effects that, if serious, would be borne by children/adolescents for potentially decades.
Given the billions that Pfizer has earned on its mRNA COVID vaccine (and other pharmaceutical products), the company certainly has the financial resources to have conducted a much larger clinical trial for the 12–15 age group, but the company chose not to. Of course, the FDA might have insisted on a larger trial; thus, the FDA is a partner in Pfizer's “crime” of not conducting an adequately sized phase III trial for 12–15 year-olds. Let's turn our attention to what may truly constitute criminal conduct engaged in by Pfizer and by those it hired to run this trial.
Maddie de Garay, then a healthy twelve-year-old, enrolled in Pfizer's trial for her 12–15 age group. She is now confined to a wheelchair. What happened to Maddie is described by her mother Stephanie as follows:
On January 20th, Maddie received her second dose of the Pfizer COVID vaccine as a participant in the clinical trial for 12 to 15 year-olds. All three of our kids volunteered and were excited to participate in the trial as a way to help us all return to normal life. … Before Maddie got her final dose of the vaccine, she was a healthy twelve year old who got straight A's and had lots of friends. … Upon receiving the 2nd shot, Maddie immediately felt pain at the injection site, and over the next 24 hours she developed severe abdominal and chest pain. The way she described the chest pain and I quote: “It feels like my heart is being ripped out through my neck.” She had painful electrical shocks down her neck and spine that forced her to walk hunched over. She had extreme pain in her fingers and toes; it actually made them turn white and they were cold whenever you touched them.
You can listen to the full description below, starting at 31:39.
Additional important information that Maddie's mother provides in this video, which was recorded 28 June 2021, includes,
In the discharge papers from the Children's Hospital ER [Cincinnati Children's Hospital] that she went to [initial emergency-room visit], the diagnosis stated “adverse effect of vaccine initial encounter.” This would be the only time that that was written in medical charts but it's in there.
On 2 November 2021, during a roundtable discussion held in Washington DC, Maddie's mother provided further testimony concerning Maddie's and her family's ordeal, from which I'll share the following excerpts.
It’s been over 9 months since she got her second dose; she can’t walk, has an NG tube for all nutrition, and has constant pain in her stomach, back, and neck. She can’t feel her legs. That’s just the tip of the iceberg.
What made it into the trial record is unclear and yes we did ask several times and have it documented. We still don't know what was actually recorded. … So, what was said about Maddie … [in] the NEJM article about the Pfizer COVID vaccine in adolescents? … There's absolutely no mention of any of Maddie's adverse reactions in the article. Zero.
In the EUA amendment, Maddie’s adverse reaction was reduced to 5 lines that they claim was eventually diagnosed as Functional Abdominal Pain. That's a stomachache. By the data cutoff for the trial on March 13, Maddie experienced over 35 adverse events. … This happened during the trial. Some examples are blood in her urine 7 times, decreased vision, loss of feeling from her waist down, dizziness, fainting, tremors, muscle weakness, and more. … None of these were mentioned in either document. She went to the ER 9 times and was hospitalized 3 times for a total of 63 days and this was by June 1st. … Maddie was in the hospital when the EUA was approved for 12–15 year-olds [May 10th, 2021].
So let me ask you this: functional abdominal pain—stomachache. Does your child, when they have a stomachache, does that put your child in a wheelchair? Does your child’s stomachache require a feeding tube? Does your child spend 64 days in a hospital for their stomachache? If they did a urine test, when they have a stomachache, would there be blood in their urine?
I thought that Maddie would be in the best hands possible in the rare chance she had a severe reaction. That was not the case. They did everything in their power to hide everything that happened to her and that is why this is happening to all these other people and kids.
If they minimized Maddie’s reaction to the vaccine like this, I wonder what really happened to those in the clinical trials; who had a reaction to the first dose? … I wonder what else was hidden in either the Pfizer trials or in any of the other ones.
Neither Pfizer, the FDA, or the CDC has ever talked to us, or attempted to. We have never heard anything from them, ever; and I did file it in VAERS also—still didn't hear anything.
Doesn't the preceding provide sufficient justification to launch a criminal investigation, designed to determine the extent to which Pfizer has conspired, with, e.g, Cincinnati Children's Hospital and the FDA, to falsify or hide from the public data from its COVID-vaccine phase III clinical trial for 12–15 year-olds? Certainly, the cover up of Maddie de Garay's vaccine injury should provide the reader sufficient justification not to trust Pfizer (and perhaps the FDA and CDC as well).
Let's analyze a claim made in the NEJM article describing results of Pfizer's COVID-vaccine clinical trial for 12–15 year-olds. The next-to-last sentence in the section of the article titled “Adverse Events” reads as follows:
Few participants in any cohort (≤ 0.4% through 1 month after dose 2) had serious adverse events, and none were considered by the investigators to have been vaccine-related.
So, the clinical-trial investigators claim that none of the serious adverse events were vaccine-related. You are now very familiar with the serious adverse event, in fact, the “health disaster” that trial participant Maddie de Garay experienced, which began during the 24-hour period following her second Pfizer COVID shot. The investigators claim that this “health disaster” is unrelated to Maddie's vaccination. Equivalently, they are claiming that this disaster happened by pure chance.
What is the probability that the investigators are correct—that during the 24-hour period following a second shot of BNT162b2, one of the trial participants in the treatment group would have experienced, by pure chance, a health disaster like Maddie's? I've estimated the probability to be at most 0.001, that is, at most 1 in 1,000. See the supplement section concluding this post: “Calculating the probability that an unusual event will happen to a treatment-group trial-participant during the 24-hour period after receiving dose 2.”
Thus, we have compelling evidence showing Pfizer's investigators' claim that Maddie's health disaster was not vaccine related is untenable. Even without the probability estimate that I've provided, I believe that no reasonable, unbiased “investigator” would have judged Maddie's health disaster to be unrelated to the vaccination shortly after which her highly unusual symptoms began to develop. Worth noting is that 8 of 10 of the authors named at the top of the NEJM trial report are on Pfizer's payroll—perhaps pleasing Pfizer, for some or all these authors, is more important than honestly assessing vaccine safety. It seems possible that some authors/investigators were not familiar with the facts of Maddie's case. Should that be a comforting thought?
Is it comforting that even though the FDA and CDC are now aware of Maddie's health disaster, these agencies, charged with promoting and protecting public health, have never contacted Maddie or her family? Stephanie de Garay was absolutely correct to expect that “Maddie would be in the best hands possible in the rare chance she had a severe reaction.” The moral, public-health, and scientific obligation to treat and investigate a potential vaccine injury occurring during a clinical trial is clear. In Maddie's case, because the FDA and CDC are essentially serving as marketing agencies for COVID vaccines, this obligation falls on these agencies as well as Pfizer.
Did you notice that I placed in boldface type one of the adverse reactions to Pfizer's COVID jab that Maddie experienced? Blood in her urine. Read the following excerpts of an article posted 3 February 2022 at the website of NBC affiliate WWLP:
According to the Bradford County [NY] Deputy Coroner, a rare complication to the COVID-19 vaccine turned deadly for the Watts family of Lockwood. Their son, George Watts Jr., died at just 24-years-old after receiving the Pfizer-BioNTech COVID-19 vaccine. … After his first dose, George Jr. experienced complications, which he chose to keep to himself. According to his parents, blood was in his urine after the first shot. He then received his second dose in mid-September, where he experienced flu-like symptoms that did not resolve. … By the end of October, his symptoms were even worse. … George Jr. collapsed in his room on October 27 and was pronounced dead later that morning. His dad described him as healthy, saying he had no underlying medical conditions. … “The cause of death is the COVID-19 vaccine-related myocarditis,” Timothy Cahill Jr., Chief Deputy Coroner for Bradford County, said. “We are currently working on other cases that are related to vaccine and booster-related issues within our county.”
Had Pfizer, the FDA, and the CDC followed up on Maddie's vaccine injuries (and those of others—see realnotrare.com) and tried to determine what went wrong, perhaps they would have noticed patterns in adverse reactions to vaccination; for instance, blood in a vaccine recipient's urine might indicate an increased likelihood of myocarditis. Maddie's health disaster began 20 January 2021, and the death of George Watts, Jr., owing to “COVID-19 vaccine-related myocarditis,” occurred over nine months later. If Pfizer, the FDA, and the CDC had done their jobs, perhaps George Jr. would be alive today.
Why is Senator Ron Johnson the only national political leader who seems to care about the moral, public-health, and scientific obligation to investigate carefully as well as provide excellent treatment of injuries sustained by clinical-trial participants? Does the mainstream press not care simply because it doesn't wish to lose advertising revenue from pharmaceutical companies?
A Presidential Commission should be appointed to investigate the extent to which “the science” was not followed in COVID-vaccine clinical trials and, equally important, to determine the extent to which the FDA and CDC have failed in their missions. Should the Commission find evidence of criminal conduct, those who engaged in that conduct should be prosecuted. The Commission should recommend ways to fix our broken public-health agencies, which appear to be serving neither science nor the public, but rather the pharmaceutical industry.
6. Is forcing one’s faith on others logical?
In the context of the preceding question, most would interpret “faith” to mean religious faith, and I'm sure nearly everyone would say no—forcing one's faith on others is not logical, it's not ethical, it's not constitutional (violating the first amendment).
I believe forcing one's faith in COVID-19 vaccines on others is also not logical, not ethical, and not constitutional. I'll address only issues of logic and ethics here.
In my view, it's more logical for healthy persons to put their faith in the human immune system* alone, rather than putting faith in the assistance provided by mRNA vaccines that
provide only modest, short-term protection from infection,
were hastily developed by potentially untrustworthy pharmaceutical companies (e.g., Pfizer) with ineffective regulatory oversight, and
have nontrivial short-term risks and unknown long-term risks.
Those who wish to mandate COVID-19 vaccines have put their faith in the vaccines (as well as the companies that manufacture them); therefore, they must believe the vaccines protect them from, at the very least, severe infection. Their faith that vaccination often leads to mild or asymptomatic cases, together with some logical thinking, should lead them to realize, as I've already indicated, that the vaccinated would therefore be more likely to spread COVID-19 because they are more likely to be out in public, infected but feeling fine, than the unvaccinated. Thus, there is no reason that is both logical and ethical for persons to force their faith in COVID vaccines on others. Why “both logical and ethical” rather than just “logical”? There is a logical reason some might wish to force their vaccine-faith on others: I wonder how many persons in positions of authority who support, recommend, or enforce vaccine mandates have investments in Pfizer and/or Moderna? Oh, how I wish Katharine Graham were still CEO of the Washington Post Company and her definition of news were every journalist's definition of news.
______________________________
*the product of millions of years of evolutionary refinement---some estimate we are 2.4 million years beyond homo habilis
Supplement: Calculating the probability that an unusual event will happen to a treatment-group trial-participant during the 24-hour period after receiving dose 2
The “unusual event” of interest here is the “health disaster”' experienced by Maddie de Garay having onset within the 24-hour period following her second dose of BNT162b2 during Pfizer's phase III trial for the 12–15 age group.
We first need to estimate the probability that, during a calendar year, a healthy 12–15 year-old child experiences a health event that, e.g., (i) causes severe chest pain (“It feels like my heart is being ripped out through my neck”), (ii) entails painful electrical shocks down the neck and spine that force the child to walk hunched over, (iii) results in extreme pain in fingers and toes, and (iv) eventually puts the child in a wheelchair. I'm sure everyone would agree this probability is quite small. How should we estimate it? Let's look of probabilities of a child's developing two different serious illnesses. The American Cancer Society says that “About 10,470 children in the United States under the age of 15 will be diagnosed with cancer in 2022.” We'll use a Statistca estimate for the population of children in the U.S. under 15: 60.57 million as of 1 July 2021. Thus, we get 10470/60570000 ≈ 0.000173 as a probability estimate that a child under 15 develops cancer in a calendar year. Maddie's condition, which, e.g., resulted in her being wheelchair bound, seems more rare than her developing one of a number of different possible cancers. Given the severe chest pain that Maddie experienced and our current knowledge of the connection between mRNA COVID vaccination and myocarditis, perhaps Maddie developed myocarditis following her vaccination. There are documented cases involving development of significant symptoms of confirmed myocarditis shortly after the second dose, including “feeling like having a heart attack 24/7.” The myocarditis foundation states, “The incidence of myocarditis in children is uncertain but it is estimated that 1 per 100,000 children per year are affected.” Given these probabilities (developing cancer in a given year 0.000173 or myocarditis 0.00001), it seems reasonable to assert that 0.000173 is an upper bound on the probability that a 12 year-old girl develops in a calendar year the highly unusual symptoms that Maddie did.
To facilitate discussion, let's call the illness that Maddie developed following her second dose of BNT162b2 “HUI” (Highly Unusual Illness). We assume the probability that a child develops HUI in a calendar year to be at most 0.000173. Let's estimate the probability that HUI symptoms appear during the 24-hour period after a second BNT162b2 dose (during the clinical trial) to be 1/365. Thus, our estimate of the probability that a child in the trial treatment group would develop HUI, by chance, during the 24-hour period following a second dose is 0.000173 × 1/365 ≈ 0.000000474. There were 1131 members in the treatment arm of the trial of whom 1119 received the second dose. We need to determine the probability that one or more of them would develop HUI symptoms by pure chance, during the 24-hour period following dose 2. Let's imagine that each of the 1119 trial participants receiving a second dose tosses a coin—on one side there's a “Y” and on the other, an “N”. “N” means “no,”, the child does not develop HUI symptoms during the 24 hours post dose 2, and “Y” means “yes” the child does develop HUI during the 24 hours post dose 2. However, this is very unusual coin: there is only a tiny chance, namely a 0.0000474% chance, the coin will flip to “Y”. The probability that all coins will flip to “N” is
The probability that this does not happen—that at least one coin turns up “Y” is 1 - 0.99947 = 0.00053, which less than 0.001. That is, the probability that one for more treatment-group trial participants would contract, purely by chance, HUI during the 24-hour period after dose 2 is less than 1 in 1,000.
Paul Bourbon is a Professor of Mathematics, General Faculty, University of Virginia (Retired); Formerly, Cincinnati Professor of Mathematics at Washington & Lee University









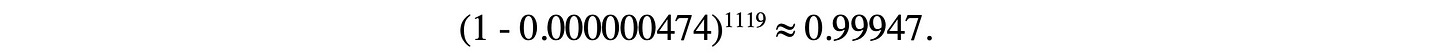
I wrote an article two years ago saying you don't have to know any "science" to reject the vaccines (especially if you are under age 45 and especially if you are a healthy child). All you need is basic math skills that would allow you to understand simple statistical probabilities. What are the odds you would die from Covid?
For a healthy child (0 to 17) with no severe pre-existing conditions, the odds you would die from Covid in the span of a 1-year time in the first year of the pandemic were about 1-in-1.95 million. This is based on an in-depth UK study that looked at the real causes of all childhood deaths in the first year of the pandemic. As a percentage, that risk is 0.0001 percent.
Only six "healthy" children in the UK died "from" Covid in the first 12 months of the pandemic - out of about 11.7 million children who did not have severe pre-existing or 'life-altering" medical conditions.
Cross-posted! Amazing work!